One of the biggest challenges of our generation will be to reverse the effects of climate change caused by the long-term increase in CO2 emissions. In theory, there are two ways to approach this problem, either by dealing with the CO2 once it has been generated (decarbonisation) or by preventing its production altogether (carbon repurposing).
Although the idea of decarbonising might initially seem appealing, it is largely impractical within the context of our daily lives. The ambition to reduce, reuse and recycle combined with lowering consumption in the first place should be strongly encouraged, however, a dogmatic ambition for ‘total decarbonisation’ is impossible against our basic human requirements for food, clothing and shelter. Despite this, a large part of Innovate UK’s Strategy Challenge Fund is being directed at carbon capture storage, which is considered as a kind of CO2 landfill exercise.
But how can we tackle this problem more cleverly?
Here at Ingenza, we have been investigating different ways of manipulating the chemical reactions involved in fermentation to reduce the production of CO2 and other greenhouse gases – or even to stop it altogether. This could be hugely beneficial in many industrial applications, for example, in the cattle industry, or in bioethanol production for the fuel and food and beverages sectors. Manipulation of redox reactions during the fermentation process isn’t a novel concept, but its translation into real life usage with long term benefits is a challenge. The redox management of carbon is based on our understanding of the underlying chemistry and energy values attributed to the different states of carbon, both in feedstock and in the products generated from it. When we burn any carbon substrate, this allows us to easily access this energy, which is why these pathways have been so prolifically exploited by man for industrial use. However, we can also manipulate these chemical reactions using renewable energy sources to completely stop the formation of CO2 as an end-product.
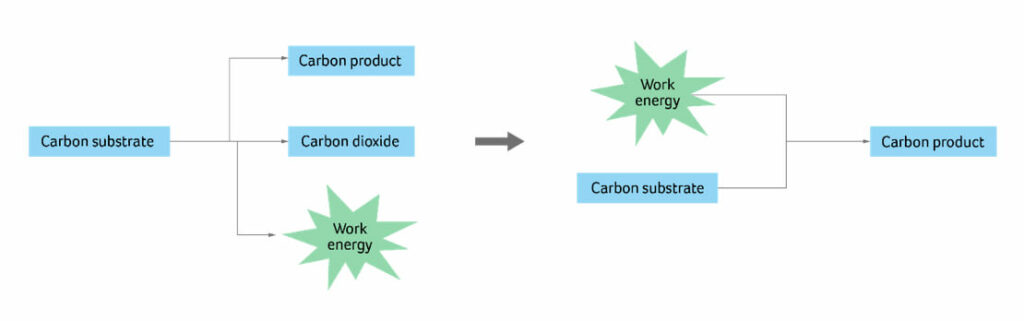
Figure 1 describes the underlying principle behind the approach we’ve taken to improving and increasing the sustainability performance of bioprocesses that already exist. Our goal is to apply this process and innovative thinking into feedstock opportunities so that we can, for example, continue our consumption of cow-derived products, with lower environmental impact.
The making of an environmentally friendly cow!
Using the cattle industry as an example, rumen fermentation takes place in the cow’s digestive system or rumen, where a microbial community converts the ingested feed into energy. Complex sugars in feedstock enter this chemical reaction and are processed into volatile fatty acids (VFAs) which are absorbed by the cow’s bloodstream, with methane being formed as a by-product. This methane is belched by the animal, and this accounts for the majority of the carbon emissions from ruminants, which contributes significantly to greenhouse gas emissions (see Figure 2).
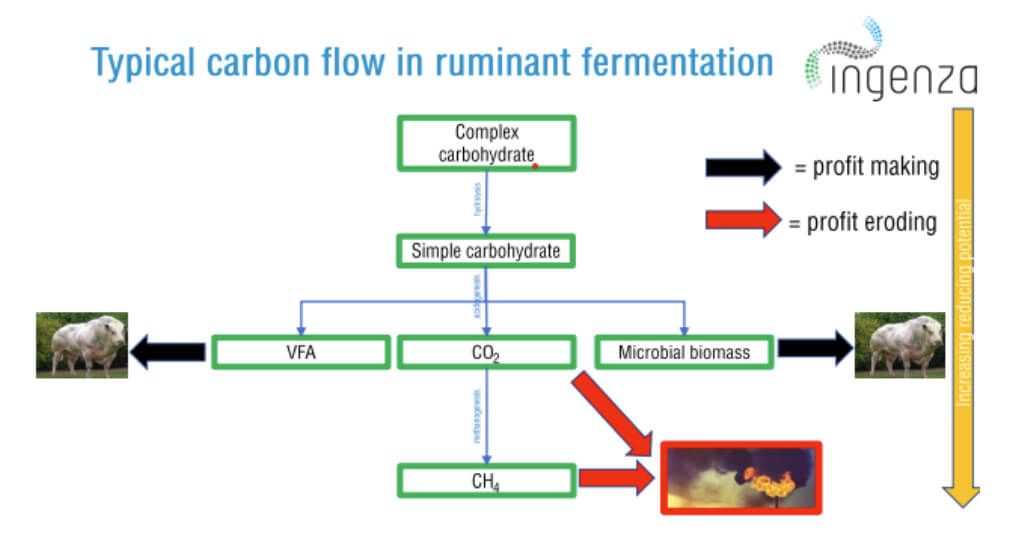
The methane emitted carries around -891 KJ/mol in energy that we cannot burn, energy that, if harnessed, could feed one and a half people per head of cattle. There is, however, a path we could explore by redirecting this chemical reaction, managing the redox potential during the rumination process, and concomitantly minimising the amount of methane formed. The important point is to exploit the chemical reaction in the fermentation process in our favour, but in a way that doesn’t add to the net cost of the feed and, more importantly, that doesn’t harm the animals and the surrounding environment.
How feasible is this technology?
Early results have been incredibly positive in reducing methane production, and we are now assessing this technology’s potential with multiple stakeholders. We’re excited to see how this application develops and look forward to using our internal chemistry expertise to find even more opportunities where manipulating redox can make a real difference to the carbon produced. Watch this space!